Medical Device Certification
Meet medical industry and customer requirements
Business Challenge
Meeting legislative regulations and customer requirements for the manufacture and sale of medical devices in a global marketplace can be confusing. With so many regulations, differing from country to country, an organization must address a complex array of quality, process, and
management issues in order to compete internationally.
Solution
ISO 13485 defines the requirements that medical device manufacturers must incorporate into their management systems in order to achieve compliance in the medical device industry. Based on ISO 9001, the emphasis on continual improvement is replaced with an emphasis on meeting regulatory and customer requirements, risk management,and maintaining effective processes, namely the safe design, manufacture, and distribution of medical devices.
It is important to note that being certified to ISO 13485 does not fulfill the requirements of either the FDA or foreign regulators; however, the certification provides a solid framework on which to build compliance to various regulatory and customer requirements.
Benefits of implementing ISO 13485
-
Potentially gain entrance into international markets
since certification to ISO 13485 is seen as a first step toward achieving compliance with European regulatory requirements.
-
Help protect your organization’s financial investment
through harmonized quality management system requirements for regulatory purposes.
-
Create a framework for implementing a systematic approach
to monitoring your organization’s processes and your customer’s feedback
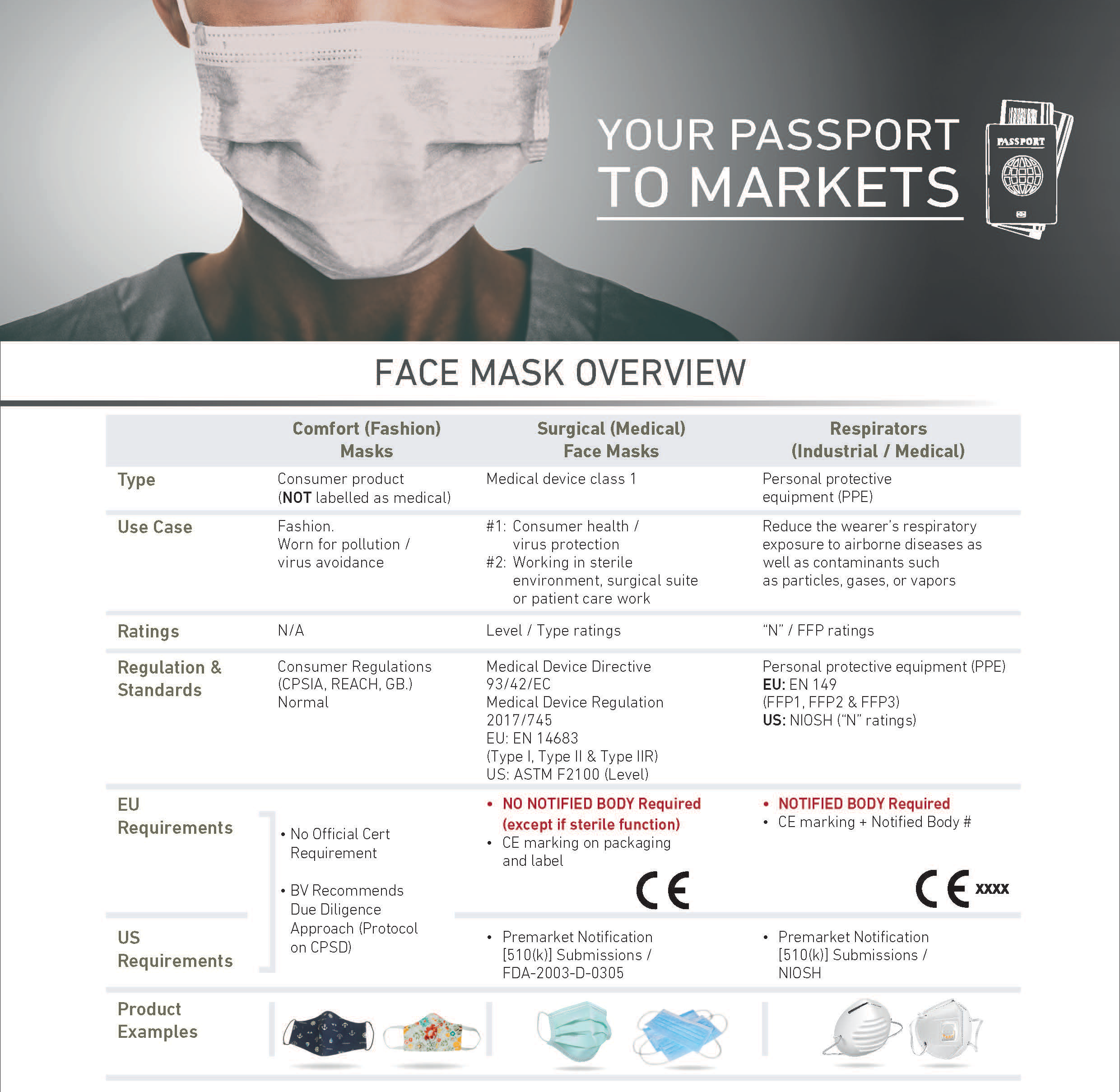
BUREAU VERITAS SERVICES RELATED TO MASKS
TESTING AND INSPECTION
Bureau Veritas has qualified inspectors in major face mask production countries worldwide. The inspection protocol addresses quantity, labeling and specification review as well as a variety of on-site testing including: product dimension measurement, nose clip reliability, tensile test to the mask connection points, claimed function as well as actual wear test.
Bureau Veritas has a global and growing network of labs in America, Europe and Asia able to perform testing for all types of face masks. Our specialist hubs worldwide can also provide specialist filtration efficiency testing against global requirements such as Europe’s EN 14683 and the USA’s ASTM F2101. By testing and inspecting your masks we support you in verifying that your product meets quality and regulatory requirements in countries worldwide.
REGULATORY AWARENESS
Understanding the regulation when you want to import or export masks from one to another country is key when you want to access a market. Bureau Veritas can help you to better understand the standards and local requirements your product needs to comply within the destination area.
Our specialists can also provide training to your personnel to get acquainted with the CE marking and other regulations.
ADMINISTRATIVE AND DOCUMENTATION REVIEW
At pre-shipment stage, we can help you verifying the adequacy of the documentation including the applicable test requirements, third party lab accreditation, vendor information and overall rating of your masks. Bureau Veritas is also specialized in verification of conformity (VOC) program of goods imported into a certain number of countries.
FACTORY AUDIT
Whether the masks are respirators or comfort masks, their manufacturing site must comply with specific requirements such as sterilization facilities, disinfection areas, clean rooms, proper protective clothing and storage. Bureau Veritas proposes dedicated site and factory audit to assess the compliance.
TECHNICAL ASSISTANCE FOR MACHINERY PRODUCING THE MASKS
Machines experience natural wear that can lead to all kinds of accidents or incidents. From the design stage through to everyday use, operator safety is a key priority. Bureau Veritas Qualified Machinery Experts can assist you during the whole life cycle of your mask manufacturing machines, from certification via periodical inspection to decommissioning.
Typically for masks manufacturers in the EU, Bureau Veritas can technically assist along the CE marking self-declaration process of the machinery (or the assembly).
SUPPLIER ASSESSMENT
As suppliers are often far from purchasers, Bureau Veritas, thanks to its global footprint, proposes a service of supplier assessment to its clients.
This service helps purchasers to verify the qualification of their potential suppliers when considering their capabilities to manufacture specified masks. It is generally 2nd Party assessment, based on customized on-site processes and focused on suppliers technical execution. The evaluation of Bureau Veritas encompasses QHSE and CSR issues at the same time to help our clients select their most appropriate suppliers. This service can be delivered for all types of masks, except for surgical face masks related to EU directives.
CERTIFICATION OF QUALITY MANAGEMENT SYSTEM
Bureau Veritas is accredited for delivering ISO 13485 certification. This international standard specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices, like medical face masks, that consistently meet customer and applicable regulatory requirements. Based on the principles of the ISO 9001 the emphasis on continual improvement is added in order to meet regulatory and customer requirements, risk management, and maintaining eective processes, namely the safe design, manufacture, and distribution of medical devices.
Adopting ISO 13485 provides a practical foundation for surgical masks manufacturers to address the EU Medical Device Directive
(MDD), the EU Medical Device Regulation (MDR), and other regulations, as well as demonstrating a commitment to the safety and quality of their masks.
It is addressed to all organizations involved in one or more stages of the masks’ life-cycle, including design and development, production, storage and distribution.
CERTIFICATION OF MASKS OR TECHNICAL ASSISTANCE TO SELF DECLARATION
In the EU, surgical face masks class 1 are under self-declaration by the manufacturer and Bureau Veritas can thus neither provide certification nor propose technical assistance. In the US surgical face masks must comply with the ASTM F2100 and Bureau Veritas can accompany the manufacturers with technical assistance. Bureau Veritas can also assist manufacturers and suppliers to demonstrate compliance and certification with Australian and New-Zealand Standards for masks and respirators according to AS 4381 and AS/NZS 1716.
For more information
Tel. +662 670 4800
E-mail: prthailand@bureauveritas.com